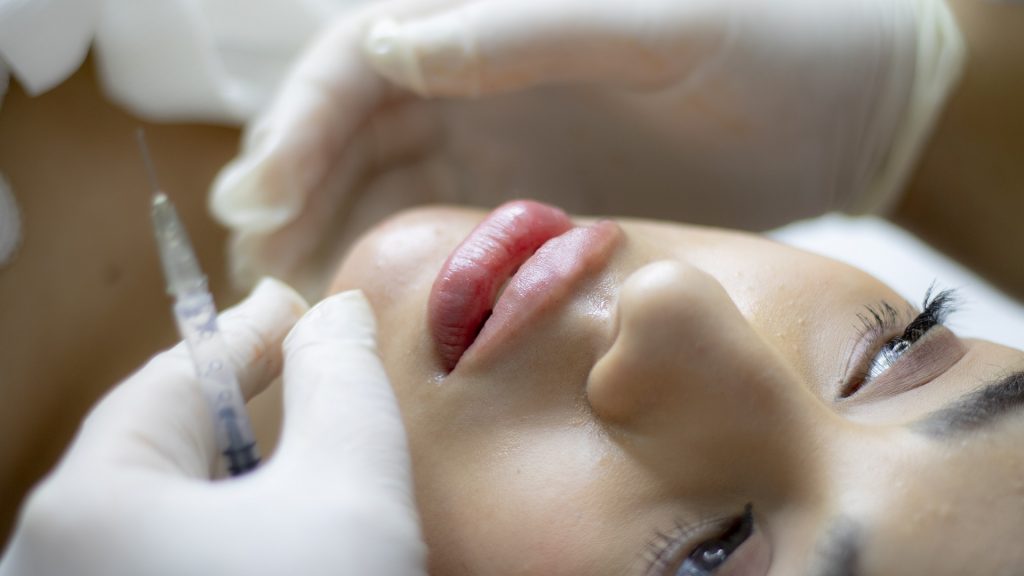
New regulations from Government to crack down on ‘botched’ botox
THE government is set to impose new licence requirements on unregulated cosmetic surgery providers in an effort to battle ‘botched botox’ procedures.
Changes to Health and Care legislation will be made on Tuesday which give the government’s health secretary powers to enforce a new licencing regime for botox and fillers.
The move follows a ban on these procedures for under 18s in England and comes amid an “unacceptable” rise in people being mentally and physically harmed following these procedures.
The new law will make it illegal for these treatments to be administered without a licence and will ban adverts on all forms of media which target under 18s.
The scope and scale of the new regulation and licence requirements are yet to be determined but the government said it will seek to introduce “consistent standards” and hygiene and safety standards.
Work is also being done by the Medicines and Healthcare products Regulatory Agency on the potential to bring certain devices, such as dermal fillers without a medical purpose, in scope of medical device regulations.