A life-changing drug has been approved by the NHS to treat babies with a rare fatal genetic disorder.
The gene therapy Zolgensma offers hope to infants with a type of severe spinal muscular atrophy (SMA).
With a list price of £1.79m, it could become the most expensive drug ever approved by the National Institute for Health and Care Excellence (Nice).
NHS England says it has negotiated an undisclosed discount that is ‘fair to taxpayers’.
The Scottish Medicines Consortium also announced later on Monday that it has approved the drug.
Around 80 babies and young children with type 1 SMA could benefit from the treatment each year in England, say experts.
The condition causes muscle weakness and affects movement and breathing, meaning most babies who have it do not live past two years without intervention.
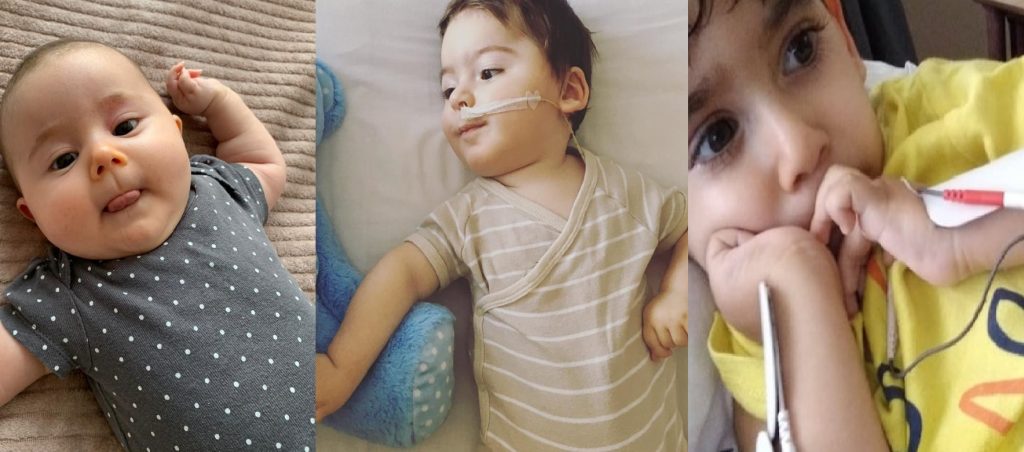
Within the Turkish Speaking community, we have seen in the last few years a number of young members being diagnosed with SMA and their family have started a crowdfunding campaign to raise the funds for the treatment outside of the UK.
In the last year, we have shared the story of Baby Metehan and Baby Ali from the UK as well as families in Turkey looking across the globe to help raise funds for the expensive treatment.
Children with the condition often need breathing assistance or a feeding tube.
The one-off treatment has been described in the British Medical Journal as “the most expensive drug course of treatment ever”.
Back in January of this year, Baby Metehan’s family reached the target of a staggering £1.9million set for his Zolgensma treatment.